Medical Product development by Amalgam to support P3 Medical’s launch of Tube Anchor
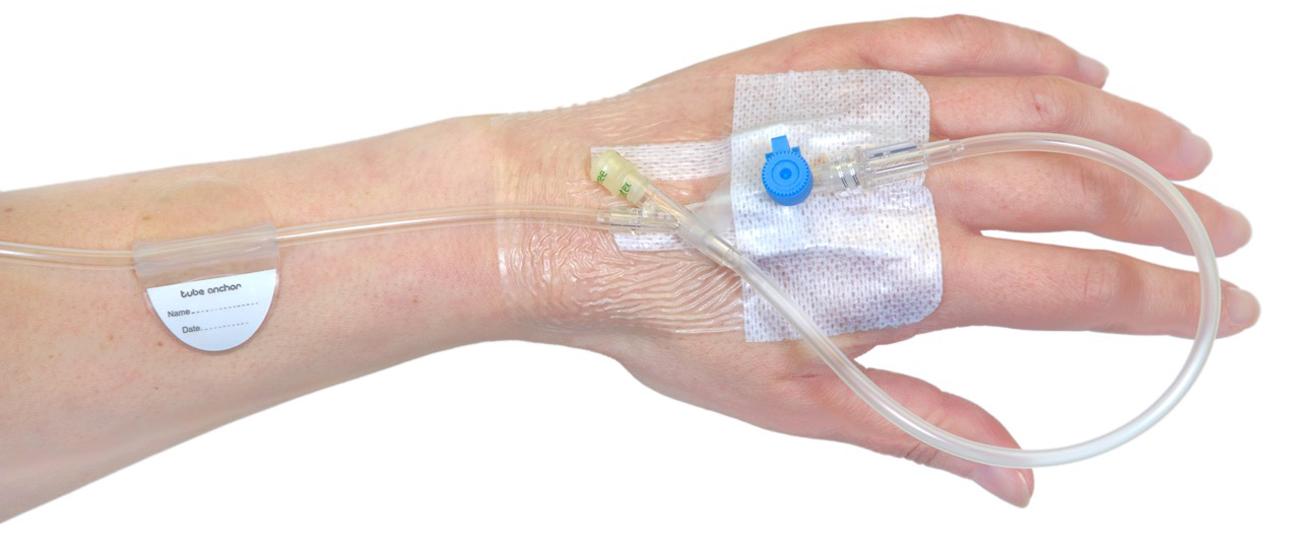
Intravenous therapy is commonplace during hospital stays, yet the way a cannula is secured means it can still tug and move, causing increased risk of infection and the need to replace the cannula frequently. Having spotted this as a commonly painful issue for patients, Professor of Endocrinology and Honorary Consultant Physician at University of Bristol, Andy Levy, came up with the idea for Tube Anchor. He recognised that a simple, disposable component could overcome this and started working on sketches and handmade prototypes in his home workshop. Very quickly he realised that fine control over materials and the geometry of the device were critical to developing the idea for wide-scale use in healthcare.
Professor Levy went on to review his plans with healthcare innovation support specialists CareTechnica, who worked with him to analyse the commercial opportunity, and introduced him to Amalgam to develop the prototype further.
“At our initial meeting Andy presented a range of ideas in sketch form and demonstrated his hand-made version to us,” says Mike Harvey, co-director of Amalgam. “Our approach for rapid prototyping is to collaborate on the design from any point in the process: we can begin from sketches or develop a product onwards from a comprehensive 3D model.”
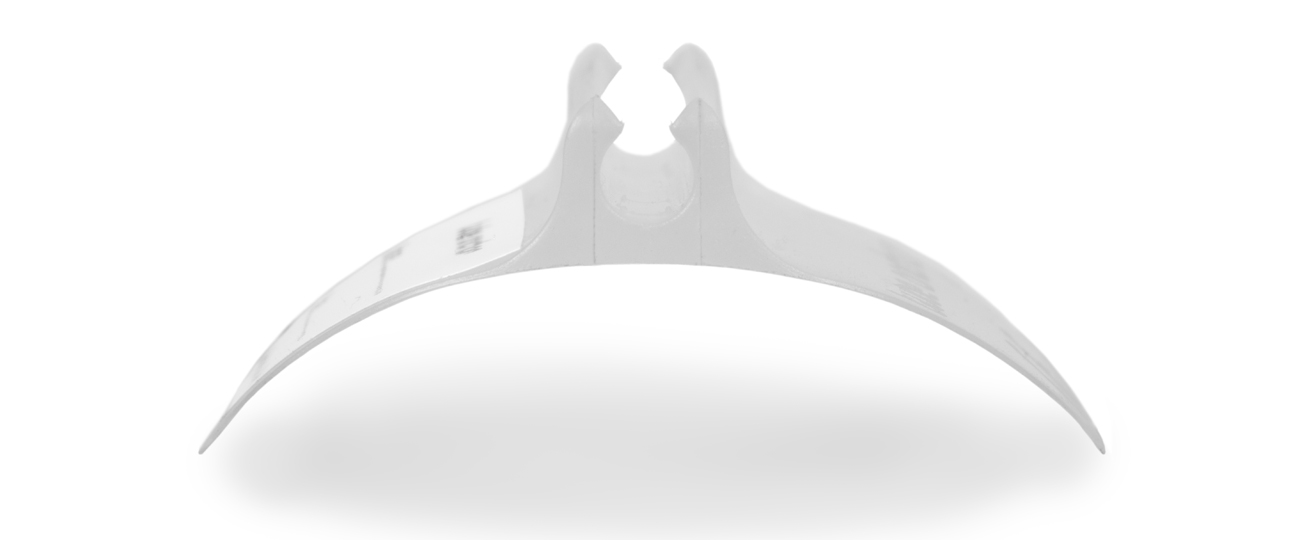
For Professor Levy, Amalgam produced several 3D model concepts in Solidworks 3D computer aided design (CAD) software to explore some of the issues, and the project quickly progressed to first-iterations being built as 3D printed models. By 3D-printing the concepts, they narrowed down how the shape of the Tube Anchor could function most effectively. The single piece design resolves many of the patient and professional issues: its’ transparent form allows a clear view of the skin underneath; it has a clean, smooth, trap-free shape for hygiene, and tubes can be removed and replaced any number of times.
Andy elaborates about the prototyping process, “It was great to sit down with Mike from Amalgam and talk through how to realise the design and create the prototypes – because he and the team really understand the complexities of injection moulding and the challenges for eventual production. From explaining my idea and initial drawings, Amalgam seemed to conjure the 3D drawings onto screen, because the shape was such a complex set of intersecting curves. The most difficult part of the design was how to produce a comfortable device to hold a very soft tube in place securely – it has to allow it in, but stop it coming out. ”
After fine-tuning, the virtual CAD models were 3D-printed, and used as master models for the casting process to allow realistic testing of materials, as well as form.
“By using the masters for soft tooling and resin vacuum casting in various elastomers, we were able to establish the physical properties the material required and produce a softer, more pliable version.” continues Mike. “It is hard to see how this product could have been refined to the degree required, without the ability to quickly and cheaply test the physical models.”
At that stage, the prototype was ready for trial with a hand-applied, commercially available, specialist skin adhesive layer. A small batch of functional prototypes were used on volunteers to gain feedback from nurses as well as the patients. With the results from the trial, Amalgam took this iteration of Tube Anchor through four cycles of fine tuning with Professor Levy, whilst CareTechnica established a licensing arrangement with P3 Medical, who now manufacture the product. Amalgam stayed close to the progress and drew up the final specifications for the tooling and production; so the team is very proud to see P3 Medical launch Tube Anchor internationally.
Martin Coulthard, Director of CareTechnica, concludes “Amalgam’s combination of digital skills, technical capability and craftsmanship, along with their ability to quickly understand the client and end-user needs, was a major factor in the success of this project.”
Andy Levy is Professor of Endocrinology and Hon Consultant Physician at Bristol University and University Hospitals Bristol NHS Foundation Trust. Bristol Medical Pro is the commercial entity for his product developments.
Tube Anchor™ European Community Design Registration Number 002571026-0001